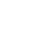Synthetic hetero-functional monomers and linkers

Synthetic hetero-functional monomers and linkers
.
"Charge-shifting" polyacrylates
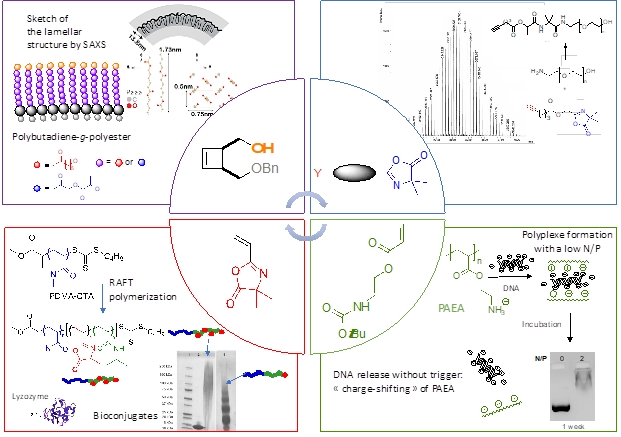
RAFT-polymerized cationic polyacrylates with tunable compositions, molar masses, and low dispersities spontaneously associate DNA to form polyplexes at a low N/P ratio. After polyplexe incubation, the “charge-shifting” behavior of polyacrylates leads to DNA release without any trigger.
Researchers
- Sagrario Pascual
- Sandie Piogé
- Véronique Montembault
- Laurent Fontaine
Related papers
- T. Ho, S. Pascual, V. Montembault, N. Casse, L. Fontaine, Polym. Chem. 2014, 5, 5542
- T. Ho, M. Le Bohec, J. Frémaux, S. Piogé, N. Casse, L. Fontaine, S. Pascual, Macromol. Rapid Commun. 2017, 38, Article Number 1600641
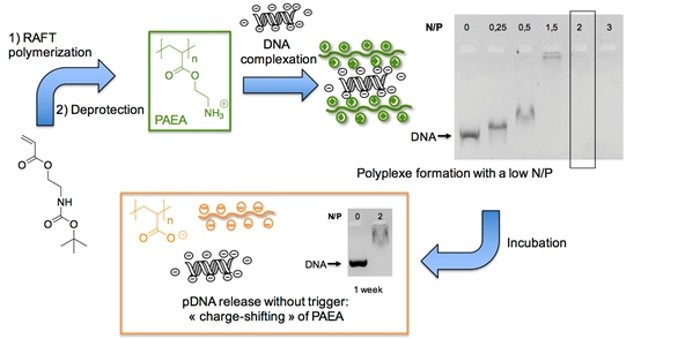
Fine-tuning of thermo-induced assembly and rheological behavior of hydrogels based on well-defined bis-hydrophilic block copolymer bearing a terpyridine entity by successive RAFT aqueous dispersion polymerization via metal ions equivalent and mixture.
Researchers
- Sagrario Pascual
- Sandie Piogé
- Lazhar Benyahia
- Laurent Fontaine
Related papers
- M. Le Bohec, M. Banère, S. Piogé, S. Pascual, L. Benyahia, L. Fontaine, Polym. Chem., 2016, 7, 6834
- A. Gutierres, S. Pascual, L. Fontaine, S. Piogé, L. Benyahia, Polym. Chem., 2018, 9, 2494
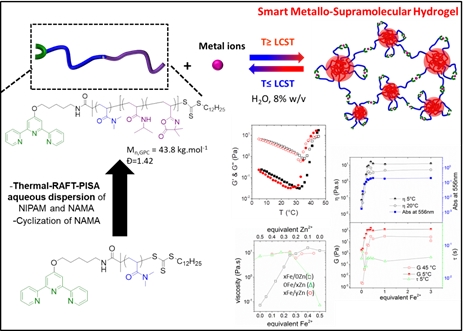
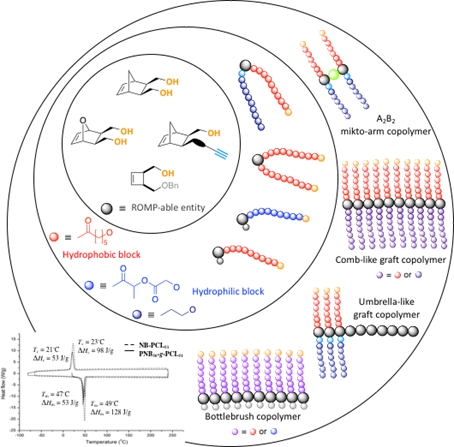
Mikto-arm, comb-like, umbrella-like, and bottlebrush copolymers via ROMP
Tunable macromolecular architectures with unique topological structures and attractive physical/chemical properties from heterofunctional precursors having orthogonal functionalities through combination of RO(M)P and Click chemistry. Copolymers with poly(oxa)norbornene or polybutadiene backbone and amphiphilic biodegradable polyesters or biocompatible poly(ethylene oxide) grafts have been designed from ROMP-able macromonomers, leading to self-organized structures.
Researchers
- Véronique Montembault
- Sagrario Pascual
- Sandie Piogé
- Laurent Fontaine
Related papers
- Le, G. Morandi, S. Legoupy, S. Pascual, V. Montembault, L. Fontaine, Eur. Polym. J. 2013, 49, 972-983.
- Le, V. Montembault, S. Pascual, F. Collette, V. Héroguez, L. Fontaine, Polym. Chem. 2013, 4, 2168-2173.
- Leroux, V. Montembault, S. Pascual, L. Fontaine, Macromolecules 2015, 48, 3843-3852.
- A. N'Guyen, F. Leroux, V. Montembault, S. Pascual, L. Fontaine, Polym. Chem. 2016, 7, 1730-1738
- Leroux, V. Montembault, S. Piogé, S. Pascual, G. Brotons, L. Fontaine, Macromolecules 2016, 49, 4739-4745
- Leroux, V. Montembault, S. Piogé, S. Pascual, L. Fontaine, Polym. Bull. 2017, 74, 4415-4422
- A. N'Guyen, V. Montembault, S. Piogé, S. Pascual, L. Fontaine, J. Polym. Sci. Part A: Polym. Chem.2017, 55, 4051-4061
Innovative heterofunctional monomers and linkers
Design of innovative hetero-functional monomers (cyclobutene, vinylazlactone, aminoethyl acrylate) as a toolbox for the elaboration of original macromolecular architectures according to controlled/living polymerization processes (RAFT polymerization, NMP, ROMP, ROP) resulting in polyfunctional polymers for potential biomedical applications.
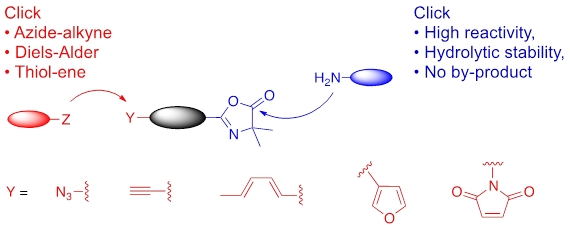
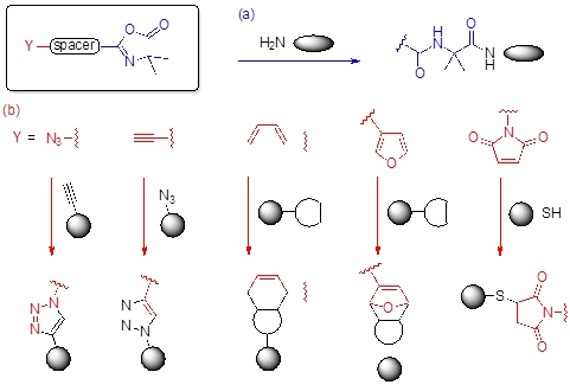
Azlactone-based heterobifunctional linkers that proceed in orthogonal click-like reactions for chemical ligations in biologically relevant medium without releasing any by-product have been successfully synthesized.
Researchers
- Sagrario Pascual
- Sandie Piogé
- Véronique Montembault
- Laurent Fontaine
Related papers
- Fontaine, H. T. Ho, S. Pascual, V. Montembault, PCT WO2014060357; US 20150285811A1; Japanese Patent n° 6262746 (2017)
- T.H. Ho, M.E. Levere, S. Pascual, V. Montembault, N. Casse, A. Caruso, L. Fontaine, Polym. Chem. 2013, 4, 675-685
- Le, G. Morandi, S. Legoupy, S. Pascual, V. Montembault, L. Fontaine, Eur. Polym. J. 2013, 49, 972-983
- T. Ho, S. Pascual, V. Montembault, N. Casse, L. Fontaine, Polym. Chem. 2014, 5, 5542
- Leroux, V. Montembault, S. Pascual, W. Guérin, S. M. Guillaume, L. Fontaine, Polym. Chem. 2014, 5, 3476-3486.
- Delplace, S. Harrisson, H. T. Ho, JA. Tardy, Y. Guillaneuf, S. Pascual, L. Fontaine, J. Nicolas, Macromolecules 2015, 48(7), 2087-2097
- T. Ho, J. Coupris, S. Pascual, L. Fontaine, T. Lequeux, T. N. Pham, Polym. Chem. 2015, 6, 4597-4604
- Leroux, V. Montembault, S. Piogé, S. Pascual, G. Brotons, L. Fontaine, Macromolecules 2016, 49, 4739-4745
- Leroux, V. Montembault, S. Piogé, S. Pascual, L. Fontaine, Polym. Bull. 2017, 74, 4415-4422
- Pray-In, C. Boonthip, B. Rutnakornpituk, U. Wichai, V. Montembault, S. Pascual, L. Fontaine, M. Rutnakornpituk, Materials Science & Engineering, C: Materials for Biological Applications 2016, 67, 285-293
- M. Le Bohec, M. Banère, S. Piogé, S. Pascual, L. Benyahia, L. Fontaine, Polym. Chem. 2016, 7, 6834
- T. Ho, M. Le Bohec, J. Frémaux, S. Piogé, N. Casse, L. Fontaine, S. Pascual, Macromol. Rapid Commun. 2017, 38, Article Number 1600641
- T. Ho, A. Bénard, G. Forcher, M. Le Bohec, V. Montembault, S. Pascual, L. Fontaine, Org. Biomol. Chem. 2018, 16, 7124-7128.
Innovative heterobifunctional azlactone-based linkers
Azlactone-based heterobifunctional linkers that proceed in orthogonal click-like reactions for chemical ligations in biologically relevant medium without releasing any by-product.
Researchers
- Sagrario Pascual
- Véronique Montembault
- Laurent Fontaine
Related papers
- Fontaine, H. T. Ho, S. Pascual, V. Montembault, PCT WO2014060357; US 20150285811A1; Japanese Patent n° 6262746 (2017)
- T. Ho, A. Bénard, G. Forcher, M. Le Bohec, V. Montembault, S. Pascual, L. Fontaine, Org. Biomol. Chem. 2018, 16, 7124-7128.
Innovative heterofunctional monomers: cyclobutene inimers
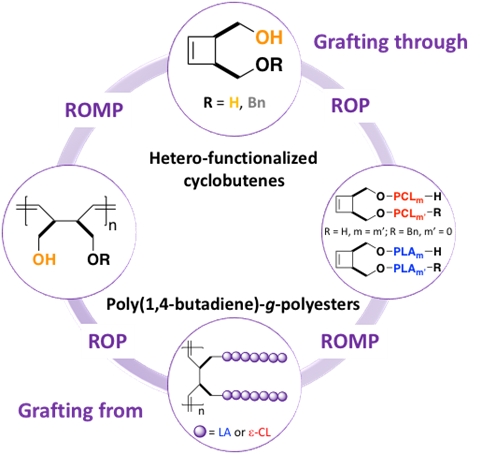
Well-defined high molar mass poly(1,4-butadiene)-g-polyesters have been designed either through the grafting from or the grafting through route by the combination of ring-opening metathesis polymerization (ROMP) and organocatalyzed ring-opening polymerization (ROP) of L–lactide (LA) and e-caprolactone (e-CL). Both methodologies provide poly(1,4-butadiene)-g-polyesters having a strictly poly(1,4-butadiene) backbone. Use of the grafting from strategy allowed the synthesis of poly(1,4-butadiene)-g-poly(e-caprolactone) (PBu-g-PCL) with the highest molar mass ever reported. Furthermore, in the solid state upon isothermal crystallization at room temperature, PCL side chains fold while they form an orthorhombic molecular network, building up a self-assembled lamellar phase with large scale d-spacing’s.
Researchers
- Véronique Montembault
- Sagrario Pascual
- Sandie Piogé
- Laurent Fontaine
Related papers
- Le, G. Morandi, S. Legoupy, S. Pascual, V. Montembault, L. Fontaine, Eur. Polym. J. 2013, 49, 972-983
- Leroux, V. Montembault, S. Pascual, W. Guérin, S. M. Guillaume, L. Fontaine, Polym. Chem. 2014, 5, 3476-3486.
- Leroux, S. Pascual, V. Montembault, L. Fontaine, Macromolecules 2015, 89, 3843-3852
- Leroux, V. Montembault, S. Piogé, S. Pascual, G. Brotons, L. Fontaine, Macromolecules 2016, 49, 4739-4745
- Leroux, V. Montembault, S. Piogé, S. Pascual, L. Fontaine, Polym. Bull. 2017, 74, 4415-4422