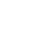New Methodologies

Méthodologie en synthèse organique
Cet axe de recherche vise le développement méthodologique pour la création de liaisons dans un environnement à forte complexité structurelle et fonctionnelle.
Directed Metalations
The metalation of arenes in the vicinity of an appropriate directing metalation group by organolithiums, lithium amides and superbases is one of the most powerful method for the regioselective preparation of synthetically useful polyfunctional arenes.
Suitable conditions have been found under which substituted azobenzenes can undergo effective and regioselective lithiation. This new reaction allows a late-stage functionalization of the azobenzene scaffold and nicely complements palladium-catalyzed regioselective functionalization reactions of aromatic azo compounds.
Researchers
- Anne-Sophie Castanet
- Anne Boussonnière
- Jacques Mortier
Related papers
- Chemoselective Deprotonative Lithiation of Azobenzenes: Reactions and Mechanisms: Thi Thanh Thuy, N.; Boussonniere, A.; Banaszak, E.; Castanet, A.-S.; Kim Phi Phung, N.; Mortier, J. Journal of Organic Chemistry 2014, 79 (6), 2775.
Diaddition of organometallics onto nitrile
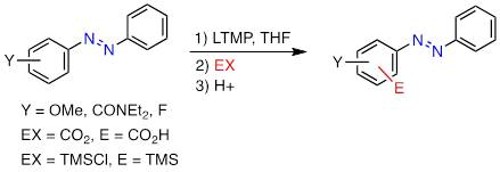
The addition of organometallics to nitriles is generally limited to a single addition, but the double addition leading to tertiary carbinamines could also occur in specific cases, such as in the chemoselective addition of Grignard reagents onto acylcyanohydrins developed in our group.
In this reaction, the selectivity of the Grignard reagents toward the nitrile moiety at the expense of the ester function is due to the proper structure of the acylcyanohydrin and is remarkable in THF. The selectivity was improved using 1-naphthoyl derivative as substrate and the addition of two different Grignard reagents was managed by the use of unreactive alkynyl organomagnesium reagent. Nevertheless, with certain Grignard reagents, some side-products resulting from the addition onto the ester drop the yield, and acylcyanohydrins displaying activated ester moiety, carbonate or other functional group present on the ester moiety could not be used.
Furthermore, the introduction of electrophilic substituent on the organometallic reagent is not feasible using organomagnesium halides. This limitation was solved by exploring the chemistry of organozinc reagent which required mild conditions and showed to be a good alternative in terms of functional group tolerance (various FG group ) and efficiency (quantitative).
Researchers
- Morwenna Pearson-Long
- Fabien Boeda
- Philippe Bertus
Related papers
- Successive addition of two different Grignard reagents to nitriles: access to α,α-disubstituted propargylamine derivatives, Caillé, F. Boukattaya, F. Boeda, M. S. M. Pearson-Long, H. Ammar, P. Bertus,Org. Biomol. Chem. 2018, 16, 1519-1526.
- Simple and convenient access to α,α,α-trisubstituted amides by double addition of Grignard reagents to acyl cyanohydrins, Boukattaya, A. Stanovych, P. Setzer, S. Abid, H. Ammar, M. S. M. Pearson-Long, P. Bertus, Chem. Commun. 2012, 48, 8655-8657. Synfacts 2012, 8(11), 1244
Enantioselective reactions
We are also interested in enantioselective reactions using organolithium organomagnesium reagents as nucleophiles.
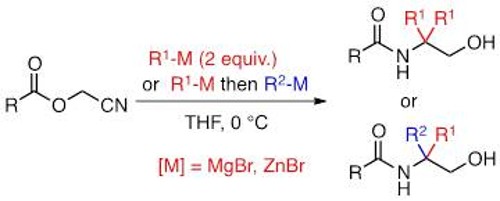
A new family of chiral 1,2-amino ethers has been efficiently synthesized in a short and scalable synthetic sequence from readily available and inexpensive α -amino or α -hydroxy acids. Stoichiometric or substoichiometric amounts of these chiral ligands can serve as effective promoters for enantioselective intramolecular carbolithiation reactions. In an effort to rationalize our experimental results, theoretical calculations using density functional theory have been conducted in order to model the course of this enantioselective reaction.
Researchers
- Anne-Sophie Castanet
- Anne Boussonnière
- Jacques Mortier
Related papers
- Readily Accessible 1,2-Amino Ether Ligands for Enantioselective Intramolecular Carbolithiation, Guyon, H.; Boussonnière, A.; Castanet, A.-S. J. Org. Chem. 2017, 82 (9), 4949.
- Transition-Metal-free Enantioselective Reactions of Organomagnesium reagents mediated by chiral ligands, Guyon, H.; Boussonnière, A.; Castanet, A.-S. Synthesis 2018, 50, 3589.
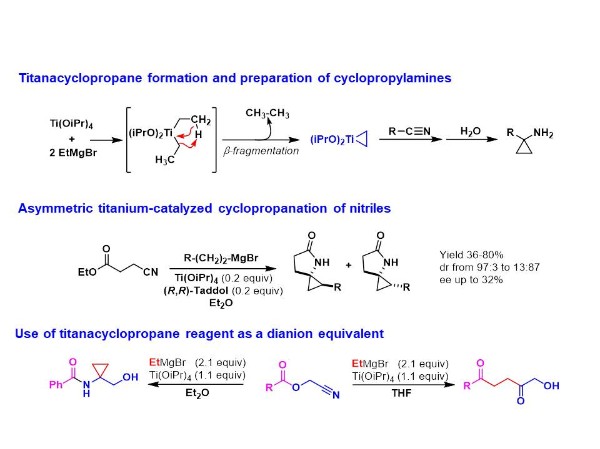
Titanacyclopropane
Since the discovery by Kulinkovich of the Ti-catalyzed conversion of esters to cyclopropanols, a direct access to cyclopropylamines from nitriles has emerged. Indeed, a mixture of Ti(OiPr)4 and ethyl magnesium bromide allows the formation of a titanacyclopropane reagent which reacts with nitriles to give cyclopropylamine compounds.
In this context, a study was devoted to asymmetric cyclopropanation of nitriles. A torough evaluation of chiral titanium catalysts has been achieved for the cyclopropanation of cyanoesters. A methodology allowing a rapid screening of chiral ligands has been developed for these transformations allowing the formation of spirocyclic compounds in good yields and with moderate enantioselectivities (up to 32%).
The reactivity of titanacyclopropane reagent towards acylcyanhydrins has led to original results. Indeed, depending on the solvent used (Et2O or THF), a,a-disubstituted hydroxyamides or 1,4-diketones can be straightforwardly prepared benefiting from the 1,2-dianion reactivity of the titanacyclopropane.
Researchers
- Morwenna Pearson-Long
- Fabien Boeda
- Philippe Bertus
Related papers
- Asymmetric Titanium-Catalyzed Cyclopropanation of Nitriles with Grignard Reagents: Caillé, P. Setzer, F. Boeda, M. S. M. Pearson-Long, P. Bertus, SynOpen 2018, 02(01), 41-49.
- Titanium-mediated addition of Grignard reagents to acyl cyanohydrins: Aminocyclopropane versus 1,4-diketones formation: Setzer, G. Forcher, F. Boeda, M. S. M. Pearson-Long, P. Bertus, Eur. J. Org. Chem. 2014, (1), 171-180.
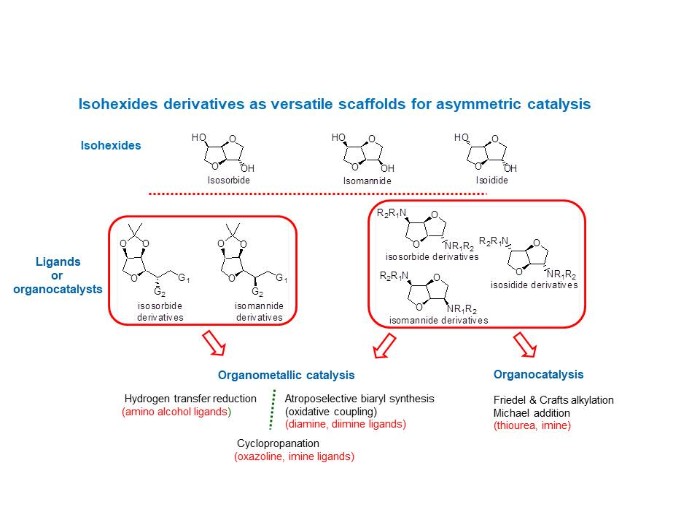
Asymmetric catalysis
Our aim is the development of tailored-made ligands and organocatalysts based on biosourced molecules: isohexides. For that purpose, we highlight two series of derivatives, the first originating from single THF ring‐opening reactions and the second preserving the original bis‐fused THF backbones. These derivatives were involved in different asymmetric reactions. Concerning organometallic catalysis, hydrogen transfer reduction of prochiral ketones was carried out with chiral Ru complexes, oxidative coupling of 2-naphtol derivatives and cyclopropanation of alkenes with ethyldiazoacetate both in presence of copper complexes. On the other other hand, organocatalysis was investigated via alkylation of indoles and Michael addition reaction.
Researchers
- Stéphane Guillarme
- Christine Saluzzo
Related papers
- Kadraoui, Mohammed; Maunoury, Thibault; Derriche, Zoubir; Guillarme, Stephane; Saluzzo, Christine European Journal of Organic Chemistry (2015), 2015(3), 441-457
- Chen, Ling-Yan; Guillarme, Stephane; Whiting, Andrew; Saluzzo, Christine ARKIVOC (Gainesville, FL, United States) (2014), (4), 215-227
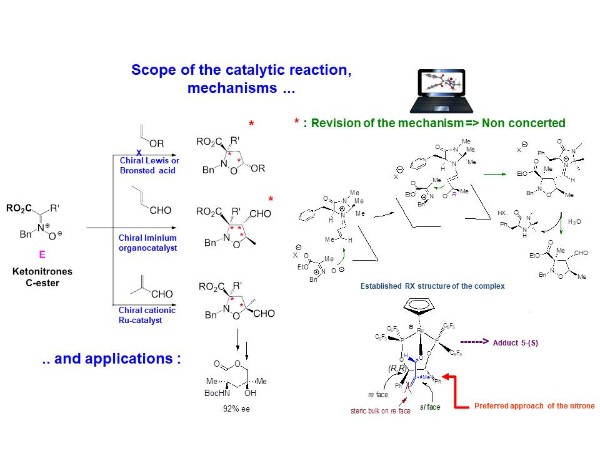
1,3-DC reactions of ketonitrones
We have extensively study the 1,3-DC reactions of new dipoles : the carboxy ketonitrones with a range of ethylenic dipolarophiles, with emphasis on the scope of the catalytic asymmetric reaction leading in a highly stereocontrolled manner to valuable multifunctional adducts, containing one or two quaternary centers.
In several cases, our dual experimental / computional study gave new insights on the mechanisms of such catalytic asymmetric 1,3-DC reactions.
Researchers
- Mathieu Laurent
- Arnaud Martel
- Gilles Dujardin
Related papers
- Cationic Ru-catalyzed of 1,3-DC reactions of carboxy Ketonitrones with metacroleïne: Selim, K.B.; Martel, A.; Laurent, M. Y.; Lhoste, J. ; Py, S.; Dujardin, G. J. Org. Chem. 2014, 79, 3414.
- Iminium-based catalyzed 1,3-DC reactions of carboxy Ketonitrones with crotonaldehyde: Ben Ayed, K. Laurent, M. Y.; Martel, A.; Selim, K. B.; Abid, S.; A.; Dujardin, G. Eur. J. Org. Chem. 2017, 79, 6763.
- Review on Isozaolidines (Synthesis and uses in medicinal chemistry): M. Berthet, T. Cheviet, G. Dujardin, I. Parrot, J. Martinez, Chem. Rev. 2016, 116, 15235.