Isohexides in asymmetric catalysis

Asymmetric catalysis
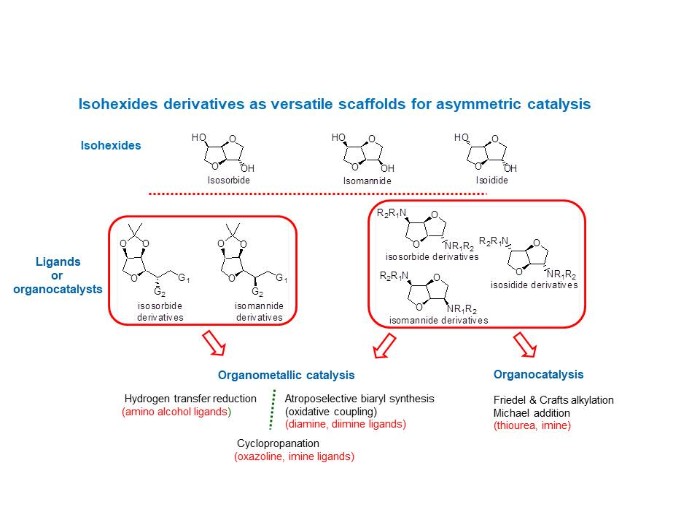
Our aim is the development of tailored-made ligands and organocatalysts based on biosourced molecules: isohexides. For that purpose, we highlight two series of derivatives, the first originating from single THF ring‐opening reactions and the second preserving the original bis‐fused THF backbones. These derivatives were involved in different asymmetric reactions. Concerning organometallic catalysis, hydrogen transfer reduction of prochiral ketones was carried out with chiral Ru complexes, oxidative coupling of 2-naphtol derivatives and cyclopropanation of alkenes with ethyldiazoacetate both in presence of copper complexes. On the other other hand, organocatalysis was investigated via alkylation of indoles and Michael addition reaction.
Researchers
- Stéphane Guillarme
- Christine Saluzzo
Related papers
- Kadraoui, Mohammed; Maunoury, Thibault; Derriche, Zoubir; Guillarme, Stephane; Saluzzo, Christine European Journal of Organic Chemistry (2015), 2015(3), 441-457
- Chen, Ling-Yan; Guillarme, Stephane; Whiting, Andrew; Saluzzo, Christine ARKIVOC (Gainesville, FL, United States) (2014), (4), 215-227