Titanacyclopropane

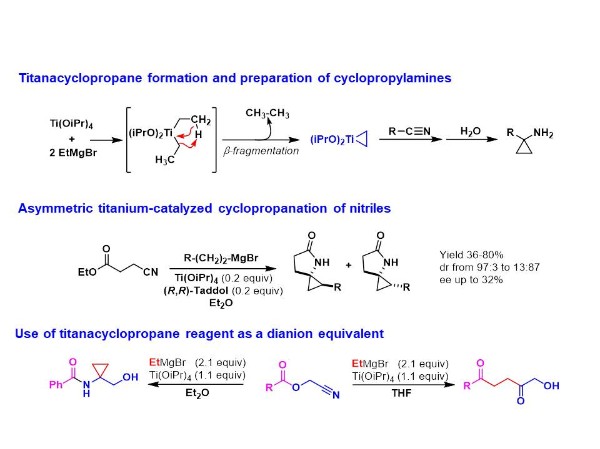
Titanacyclopropane
Since the discovery by Kulinkovich of the Ti-catalyzed conversion of esters to cyclopropanols, a direct access to cyclopropylamines from nitriles has emerged. Indeed, a mixture of Ti(OiPr)4 and ethyl magnesium bromide allows the formation of a titanacyclopropane reagent which reacts with nitriles to give cyclopropylamine compounds.
In this context, a study was devoted to asymmetric cyclopropanation of nitriles. A torough evaluation of chiral titanium catalysts has been achieved for the cyclopropanation of cyanoesters. A methodology allowing a rapid screening of chiral ligands has been developed for these transformations allowing the formation of spirocyclic compounds in good yields and with moderate enantioselectivities (up to 32%).
The reactivity of titanacyclopropane reagent towards acylcyanhydrins has led to original results. Indeed, depending on the solvent used (Et2O or THF), a,a-disubstituted hydroxyamides or 1,4-diketones can be straightforwardly prepared benefiting from the 1,2-dianion reactivity of the titanacyclopropane.
Researchers
- Morwenna Pearson-Long
- Fabien Boeda
- Philippe Bertus
Related papers
- Asymmetric Titanium-Catalyzed Cyclopropanation of Nitriles with Grignard Reagents: Caillé, P. Setzer, F. Boeda, M. S. M. Pearson-Long, P. Bertus, SynOpen 2018, 02(01), 41-49.
- Titanium-mediated addition of Grignard reagents to acyl cyanohydrins: Aminocyclopropane versus 1,4-diketones formation: Setzer, G. Forcher, F. Boeda, M. S. M. Pearson-Long, P. Bertus, Eur. J. Org. Chem. 2014, (1), 171-180.