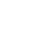Axis 1 : Investigative Crystal Chemistry and Modeling

Investigative crystal chemistry and modeling
Axis 1: Investigative crystal chemistry and modeling
Action 1.1: Investigative Crystal Chemistry
This central action for the MI thematic feeds the other actions. In this action as in the others, our knowledge in crystal chemistry and our skills in synthesis and crystallography are mobilized to discover and describe new compounds with potentially interesting properties. In these structural studies, electron diffraction (microscopy), solid state NMR, Mossbauer (J.-M. Grenèche, PSC) and Raman (A. Bulou, PSC) spectroscopies and even frequency doubling (D. Mounier, PSC) are used in addition to X-ray diffraction (laboratory or synchrotron) or neutron diffraction.
New fluorinated coordination polymers (CPs) with variable porosity are developed by combining an inorganic metallic entity with organic azole ligands. Several works have highlighted the key synthesis parameters influencing the class, dimensionality, composition and topology of the crystal edifices of these PCs [1–5]. If the use of monocyclic azole ring molecules, mostly commercialized, leads to PCs with moderate porosities (5 to 25% by volume), some of them develop unexpected properties, especially in magnetism and optics [6–9]. The size of the cavities could be increased by the use of original ligands with multiple tetrazole rings synthesized in collaboration with G. Dujardin of the SO thematic. These new fluorinated architectures (Fig. 1) built with Fe2,3+ and/or Zn2+ cations and the H2bdt ligand show remarkable porosities (up to 60% by volume) approaching those of reference materials (MIL-53, for example).
|
|
|
Fig. 1 Perspective views of two MOF structures with H2bdt as ligand. |
The work was partly devoted to the investigation and characterization of new compounds likely to be O2- conductive because of the combination of cations with high polarizability (La) and high valence (Nb, Mo or W). The careful exploration of the La2O3-MoO3 binary and La2O3-Nb2O5-(Mo,W)O3 ternary diagrams allowed the identification and structure determination of three new phases La34Mo8O75¤9 [10], La5NbMo2O16 ([11], Fig. 2) and La3NbWO10 [12]) whose crystal structures deriving from the fluorite CaF2. Although, these 3 phases exhibit a modest anionic conductivity, their discovery testifies to the relevance of the reasoned approach of these investigations.
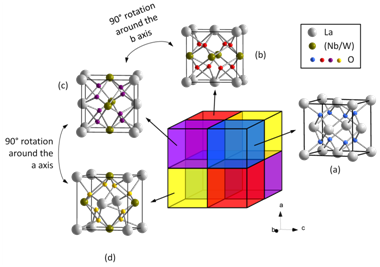
Fig. 2 Structural relation of La5NbMo2O16 with CaF2 structural type.
The existence of cations with two oxidation states in the octahedra of the columnar perovskite-like structure of La7W6+4M5+3O30 (with M= Nb and Ta) led to the idea of new compositions (La,Pr)7W6+7-xMm+xO30 in which the cation M would have a valence m= 4, 3 or 2. Such compositions have been prepared and their structures determined [13,14]. The M cations occupy the octahedra in the center of each hexagonal section column (Fig. 3) inducing a distortion of the octahedra [WO6] at the column periphery. This cationic distribution and distortion are comparable to those found in the octahedra inside and at the periphery of the sheets of the AnBnO3+2n oxide series (Fig. 3). It has been proposed to describe the 2D structures AnBnO3+2n and 1D A7B7O30 structures as resulting from a cutting, respectively into sheets and columns, of the 3D ABO3 structure that an oxygen insertion accompanies (Fig. 3).
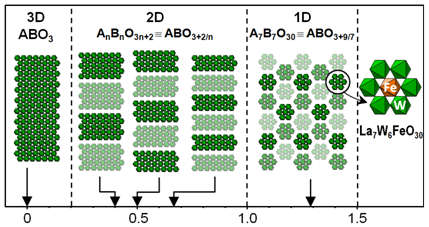
Fig. 3 Oxygen excess d in ABO3+d.
The structure of the lamellar perovskite Li2CaTa2O7, a compound that otherwise exhibits good photo-catalytic properties at room temperature, has been re-examined [15]. Diffraction techniques revealed that it is in fact non-centrosymmetric (space group Pna21) at room temperature. Two reversible structural transitions were also revealed (Pna21 -> Pnma -> Cmcm). They occur respectively around 220°C and 650°C and involve the concerted rotation and tilting of the [TaO6] octahedra within the [Ta2O7]4- sheets of the structure. These rotations/tiltings are at the origin of the disappearance of the non-centrosymmetry of the structure at 220°C, which has been confirmed by frequency doubling measurements at temperature.
Action 1.2: Modeling
Following the regional project RMN3MPL (2019-2013), we have continued to apply the GIPAW method to inorganic fluorides [16–18] but also to ordered [19] and disordered [20] oxyfluorides and hybrids [8], in the framework of a thesis [21] and of three collaborative studies. The DFT approach, i.e. Density Functional Theory, for the precise determination of NMR parameters of periodic systems, applied to inorganic fluorides since 2002 at Le Mans, has indeed become almost indispensable in solid state NMR to interpret and attribute to crystallographic sites the NMR lines of complex spectra.
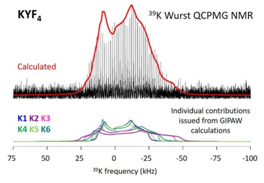
The interest of the modelling is illustrated by the 39K spectrum of KYF4 which does not allow to determine the NMR parameters of the six potassium sites. On the other hand, it is satisfactorily reproduced by the calculated spectrum (Fig. 4) showing the accuracy of the DFT calculations of the NMR parameters and thus of the structure [18].
|
|
|
|
Fig. 4. Experimental (black) and theoretical (red) 39K NMR (17.6 T) static spectra of KYF4. |
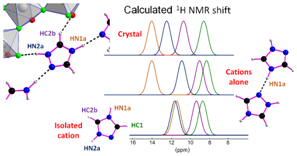
Fig. 5. Calculated individual 1H NMR resonances of [H2taz]+ cations in the [H2taz]2-(Ti5O5F12) crystal structure, alone and isolated. |
On the other hand, assigning NMR lines to crystallographic sites is necessary when one wishes to identify or study the structural factors influencing the NMR parameters of a nucleus such as the isotropic chemical shift of 89Y, particularly sensitive to the number of potassium atoms present in the second sphere of coordination for compounds of the binary KF-YF3 [18].
The "NMR crystallography" approach has been applied to two hybrid titanium oxyfluorides for which, the comparison of the chemical shifts of the protons calculated for the complete structure, for the organic cations alone and for an isolated cation (Fig. 5) has allowed to evaluate the hydrogen bonds, by adapting a method applied until now exclusively to organic solids [8].
References
(1) Abdi, I.; Lhoste, J.; Leblanc, M.; Maisonneuve, V.; Grenèche, J.-M.; Viau, G.; Ben Ali, A. [H2amtaz]+ Iron Fluorides: Synthesis, Crystal Structures, Magnetic and Mössbauer Studies. Journal of Fluorine Chemistry 2015, 173, 23–28. https://doi.org/10.1016/j.jfluchem.2015.01.017.
(2) Pimenta, V.; Lhoste, J.; Hémon-Ribaud, A.; Leblanc, M.; Grenèche, J.-M.; Jouffret, L.; Martel, A.; Dujardin, G.; Maisonneuve, V. Evidence of New Fluorinated Coordination Compounds in the Composition Space Diagram of FeF3/ZnF2-Hamtetraz-HFaq System. Crystal Growth and Design 2015, 15 (9), 4248–4255. https://doi.org/10.1021/acs.cgd.5b00530.
(3) Pimenta, V.; Le, Q. H. H.; Hemon-Ribaud, A.; Leblanc, M.; Maisonneuve, V.; Lhoste, J. Effect of the Synthesis Temperature on the Dimensionality of Hybrid Fluorozincates. Journal of Fluorine Chemistry 2016, 188, 164–170. https://doi.org/10.1016/j.jfluchem.2016.07.003.
(4) Smida, M.; Lhoste, J.; Dammak, M.; Hemon Ribaud, A.; Leblan, M.; Maisonneuve, V. Synthesis, Crystal Structure and Thermal Behaviour of a New Threedimensional Hybrid Fluoride Framework with Mixed Valence: (Fe2+/Fe3+). Chem Sci J 2017, 08 (03). https://doi.org/10.4172/2150-3494.1000161.
(5) Pimenta, V.; Oger, M.; Salek, G.; Hemon-Ribaud, A.; Leblanc, M.; Dujardin, G.; Maisonneuve, V.; Lhoste, J. Solvent Effect on 3D Topology of Hybrid Fluorides: Synthesis, Structure and Luminescent Properties of Zn(II) Coordination Compounds. Journal of Fluorine Chemistry 2018, 206, 48–53. https://doi.org/10.1016/j.jfluchem.2017.12.005.
(6) Pimenta, V.; Le, Q. H. H.; Clark, L.; Lhoste, J.; Hémon-Ribaud, A.; Leblanc, M.; Grenèche, J.-M.; Dujardin, G.; Lightfoot, P.; Maisonneuve, V. New Iron Tetrazolate Frameworks: Synthesis, Temperature Effect, Thermal Behaviour, Mössbauer and Magnetic Studies. Dalton Transactions 2015, 44 (17), 7951–7959. https://doi.org/10.1039/c5dt00281h.
(7) Albino, M.; Clark, L.; Lhoste, J.; Payen, C.; Grenèche, J.-M.; Lightfoot, P.; Maisonneuve, V.; Leblanc, M. A Magnetisation and Mössbauer Study of Triazole (M1-:X2+Mx3+)M3+F5(H Taz)1- x(Taz)x Weberites (M = Fe, Co, Mn, Zn, Ga, V). Dalton Transactions 2017, 46 (16), 5352–5362. https://doi.org/10.1039/c7dt00587c.
(8) Albino, M.; Body, M.; Legein, C.; Hémon-Ribaud, A.; Leblanc, M.; Maisonneuve, V.; Lhoste, J. NMR Crystallography, Hydrogen Bonding and Optical Properties of the Novel 2D Hybrid Oxyfluorotitanate [H2 Taz]2·(Ti5O5F12). Crystal Growth and Design 2018, 18 (11), 6873–6884. https://doi.org/10.1021/acs.cgd.8b01085.
(9) Clark, L.; Albino, M.; Pimenta, V.; Lhoste, J.; Da Silva, I.; Payen, C.; Grenèche, J.-M.; Maisonneuve, V.; Lightfoot, P.; Leblanc, M. Strong Magnetic Exchange and Frustrated Ferrimagnetic Order in a Weberite-Type Inorganic-Organic Hybrid Fluoride. Philosophical Transactions of the Royal Society A: Mathematical, Physical and Engineering Sciences 2019, 377 (2149). https://doi.org/10.1098/rsta.2018.0224.
(10) Vu, T. D.; Krichen, F.; Barre, M.; Coste, S.; Jouanneaux, A.; Suard, E.; Fitch, A.; Goutenoire, F. Ab Initio Structure Determination of La 34 Mo 8 O 75 Using Powder X-Ray and Neutron Diffraction Data. Crystal Growth & Design 2019, 19 (11), 6074–6081. https://doi.org/10.1021/acs.cgd.8b01552.
(11) Vu, T. D.; Krichen, F.; Barre, M.; Busselez, R.; Adil, K.; Jouanneaux, A.; Suard, E.; Goutenoire, F. Crystal Structure and Ion Conducting Properties of La5NbMo2O16. Journal of Solid State Chemistry 2016, 237, 411–416. https://doi.org/10.1016/j.jssc.2016.01.005.
(12) Vu, T. D.; Barre, M.; Adil, K.; Jouanneaux, A.; Suard, E.; Goutenoire, F. Investigation of the La2O3–Nb2O5–WO3 Ternary Phase Diagram: Isolation and Crystal Structure Determination of the Original La3NbWO10 Material. Journal of Solid State Chemistry 2015, 229, 129–134. https://doi.org/10.1016/j.jssc.2015.05.022.
(13) Lacorre, P. On the Crystal Structures of Pr 7 W 6.25 M 0.75 O 30 (M = Zn, Co) and so-Called MPr 2 W 2 O 10 (M = Co, Mn, Cd). New J. Chem. 2019, 43 (15), 5662–5665. https://doi.org/10.1039/C9NJ00548J.
(14) Lacorre, P.; Corbel, G. Extension of the A n B n O 3 n +2 Slicing/Oxidizing Process from 2D Layered to 1D Columnar Perovskites: The La 7 W 7-x M x O 30 Structural Family with Di-, Tri-, and Tetravalent Transition Metal M Substitutes to Tungsten. Inorg. Chem. 2019, 58 (7), 4289–4299. https://doi.org/10.1021/acs.inorgchem.8b03415.
(15) Galven, C.; Mounier, D.; Bouchevreau, B.; Suard, E.; Bulou, A.; Crosnier-Lopez, M.-P.; Berre, F. L. Phase Transitions in the Ruddlesden–Popper Phase Li 2 CaTa 2 O 7 : X-Ray and Neutron Powder Thermodiffraction, TEM, Raman, and SHG Experiments. Inorg. Chem. 2016, 55 (5), 2309–2323. https://doi.org/10.1021/acs.inorgchem.5b02659.
(16) Martineau, C.; Allix, M.; Suchomel, M. R.; Porcher, F.; Vivet, F.; Legein, C.; Body, M.; Massiot, D.; Taulelle, F.; Fayon, F. Structure Determination of Ba 5 AlF 13 by Coupling Electron, Synchrotron and Neutron Powder Diffraction, Solid-State NMR and Ab Initio Calculations. Dalton Trans. 2016, 45 (39), 15565–15574. https://doi.org/10.1039/C6DT02454H.
(17) Martel, L.; Capelli, E.; Body, M.; Klipfel, M.; Beneš, O.; Maksoud, L.; Raison, P. E.; Suard, E.; Visscher, L.; Bessada, C.; Legein, C.; Charpentier, T.; Kovács, A. Insight into the Crystalline Structure of ThF 4 with the Combined Use of Neutron Diffraction, 19 F Magic-Angle Spinning-NMR, and Density Functional Theory Calculations. Inorganic Chemistry 2018, 57 (24), 15350–15360. https://doi.org/10.1021/acs.inorgchem.8b02683.
(18) Dabachi, J.; Body, M.; Dittmer, J.; Rakhmatullin, A.; Fayon, F.; Legein, C. Insight into the Factors Influencing NMR Parameters in Crystalline Materials from the KF–YF 3 Binary System. Dalton Transactions 2019, 48 (2), 587–601. https://doi.org/10.1039/C8DT03241F.
(19) Dabachi, J.; Body, M.; Dittmer, J.; Fayon, F.; Legein, C. Structural Refinement of the RT LaOF Phases by Coupling Powder X-Ray Diffraction, 19 F and 139 La Solid State NMR and DFT Calculations of the NMR Parameters. Dalton Transactions 2015, 44 (47), 20675–20684. https://doi.org/10.1039/C5DT04028K.
(20) Dabachi, J.; Body, M.; Galven, C.; Boucher, F.; Legein, C. Preparation-Dependent Composition and O/F Ordering in NbO 2 F and TaO 2 F. Inorganic Chemistry 2017, 56 (9), 5219–5232. https://doi.org/10.1021/acs.inorgchem.7b00355.
(21) J. Dabachi. Etude Par RMN Du Solide Multi-Noyaux et Modélisation Des Paramètres RMN de Fluorures et d’oxyfluorures Inorganiques, Université du Maine, Le Mans, 2017.