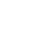Axis 2 : Materials for energy

Materials for energy
Axis 2: Materials for energy
Action 2.1: Fluorinated materials for energy
The unique properties of fluorine are at the origin of remarkable fluorinated materials that will be assets in the framework of the energy transition and the respect of sustainable development. Our research is based on the discovery of new chemical compositions via reasoned methodologies such as the decomposition of fluorinated precursors or topotactic oxidation. In order to exacerbate the properties of our materials for applications in the fields of batteries, catalysis, electrocatalysis and photovoltaics, our expertise has been extended to their nanostructuring in the form of nanoparticles and organized porous inorganic fluorides (FIPO) but also by their deposition in thin films.
The activity in the field of batteries is based on an incremental research of new cathode materials for Li or Na ion batteries. The thermal decomposition of original precursors has given access to a wide range of new fluorinated materials: crystallized hydroxyfluorides FeF3-x(OH)x of pyrochore and HTB structure and amorphous oxyfluorides with mixed cations M2+Fe3+2F8-2xOx and M2+Fe3+F5-2xOx (M = Co, Ni, Cu, Mn) (Fig. 6, left). Some of these compounds reveal properties of interest as active compounds of positive electrodes for Li+ ion batteries [1–3]. The ANR FLUOBAT 2012-17 project was part of a technological breakthrough on electrochemical storage with the development of an all-solid fluoride ion battery and the identification of an optimal electrolyte (tysonite type) with respect to ionic conduction (Fig. 6, right) [4–7]. The next step of this work concerns the PVD processing of very dense thin films of this electrolyte with a clear improvement of the intrinsic conductivity properties, close to those of a single crystal.
|
|
|
|
Fig. 1. Electrochemical properties of lithium insertion in pyrochlore and HTB iron hydroxyfluorides (left) and representation of the tysonite-like structure (right). | |
The first mesoporous OPIFs, prepared, in collaboration with of the POL thematic, by a patented method [8], have demonstrated significant potential in heterogeneous catalysis given their superior ability to withstand the extreme conditions of the catalytic fluorination reaction under gaseous HF compared to nanoparticles [9]. This work is being continued in the context of the ANR PRCE OPIFCat project (2021-24) coordinated by a member of the thematic.
It was recently established that mixed oxyfluorides based on (Co,Ni)/Fe present equivalent performances to reference materials (Ir, Ru) of anode for the water oxidation reaction [10]. The innovative character of this work has been rewarded by an Emergence project (INC-CNRS, 2020), a Samuel de Champlain Program (Conseil franco-québécois de coopération universitaire) and an International Emerging Action (CNRS 2022). The intellectual protection of these new electrocatalysts is in progress and their valorisation will performed for 18 months from 2022 in the framework of a CNRS pre-maturation project.
|
|
|
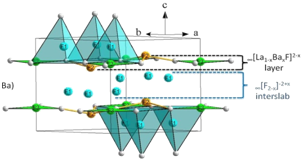
Fig. 2. Efficiency of a solar cell covered by the xMn2+-0.75Tm3+ doped ZZBAY glass |
Finally, the UV-blue IR frequency conversion on fluorinated glasses/vitroceramics with different dopants (Pr3+, Tm3+, Cr3+)/Yb3+, Tm3+, Mn2+/Tm3+ has been measured [11–16], with results to be consolidated (Fig. 2)
Action 2.2: Oxides for energy
Cationic diffusion between solid oxide fuel cell core materials is an issue that occurs both during device fabrication and operation due to the high temperatures used in both cases. A reactivity between the oxide ion conductor La2Mo2O9 (LM) and the cathode material La0.8Sr0.2MnO3-d (LSM) exists and its origin had to be determined. The cationic diffusion at the LM/LSM interface was studied by X-ray diffraction and FIB-SIMS (Imperial College collaboration, London, UK). Various reaction products were identified as well as the reaction mechanisms leading to them [17]. The highest diffusion coefficient by far is that of Mo in LSM, making the use of La2Mo2O9 as a battery electrolyte inappropriate without an effective buffer layer [18].
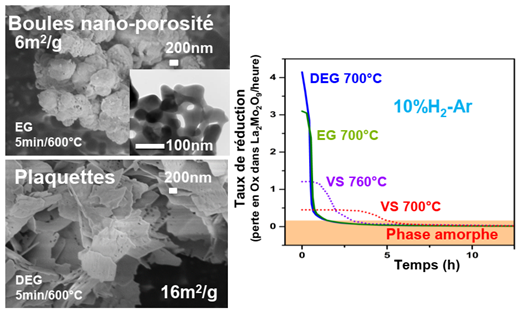
Fig. 3. Adjustment of synthesis parameters using a polyol route for fabricating La2Mo2O9
The adjustment of the parameters of the synthesis of La2Mo2O9 by a polyol route allowed to obtain without grinding or pollution nano-structured powders (Fig. 3) of specific surface 24 m2/g and dense pellets (compactness 95%) with little modified ionic conductivity [19]. The reduction kinetics of the powders obtained by solid-state synthesis and polyol (Diethylene Glycol, Ethylene Glycol) were studied up to the mixed conductive amorphous phase La2Mo2O6.7 (Fig. 3, [20]), whose local order [21]) was determined by EXAFS (joint work with Centro Atomico Bariloche, Argentina). The performance of this phase as an anode material in innovative fuel cells [22] has been measured (Joint work with Institut Jean Rouxel, Nantes).
The already very low thermal conductivity of La2Mo2O9 could be decreased by about 15(4)% by replacing half of the lanthanum by praseodymium (0.9 W.m-1K-1 at 700°C – joint work with GREMAN, Tours), making LaPrMo2O9 an interesting candidate as a thermal barrier [23].
|
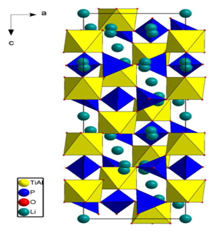
Fig. 4. Structure of Li1.3Al0.3Ti1.7(PO4)3 |
A modified Pechini method has been developed in order to obtain one of the best Li+ based conductors at room temperature, the compound Li1.3Al0.3Ti1.7(PO4)3 (LATPO) with NASICON structure [24]. NMR study of LATPO by 31P, 27Al, and 7Li nuclei revealed that the octahedra of the NASICON backbone were statistically occupied by Al3+ and Ti4+ ions (Fig. 4, [24]) and that all Li+ ions had the same environment [25]. Migration of Li+ ions into the structure requires passage through triangular oxygen windows bounded by [(Ti,Al)O6] octahedra and [PO4] tetrahedra of the framework. NMR showed that the local and coordinated distortions of the (Al,Ti)2(PO4)3 framework [26] facilitated their crossing by Li+ ions in LATPO, thus explaining its excellent conductivity. This work is the last one of our colleague J. Emery (Pr. emeritus until 2015) who performed numerous NMR studies of Li+ ion conductors in the subject.
Action 2.3: Solid state NMR characterization
Solid state NMR has been used to characterize the structure and eventually the disorder and dynamics, mainly of energy materials, through three projects or collaborations:
|
|
|
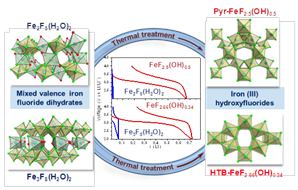
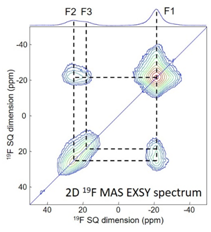
Fig. 5. 19F EXSY spectrum of La0.97Ba0.03F2.97. |
1) The ANR FLUOBAT project (see action 2.1) for which 19F NMR was a valuable tool since it allowed to characterize the environment and mobility of fluoride ions in solid electrolytes La1-xBaxF3-x [4,6,27] (Fig. 5), Sm1-xCaxF3-x [5] and Ce1-xSrxF3-x [7].
2) It was also important to quantify fluorine and to identify and quantify the various environments of fluoride ions in a hydroxyfluorinated anatase whose cationic sub-lattice is deficient, before but also after insertion of lithium, magnesium and aluminium [28–35].
3) The collaboration initiated more than 10 years ago with N. Mercier (MOLTECH Anjou - UMR 6200 CNRS, University of Angers) by the study of a hybrid perovskite, continued with the study by NMR of the paramagnetic solid of zwitterions hydrated or not [36] and associated to an inorganic part [37]. It is intensified with the study of hybrid perovskites as material of the active layer of photovoltaic cells, within the framework of the ANR MORELESS project (More stable and less lead for perovskite solar cells) [38,39].
References
(1) Lemoine, K.; Zhang, L.; Grenèche, J.-M.; Hémon-Ribaud, A.; Leblanc, M.; Guiet, A.; Galven, C.; Tarascon, J.-M.; Maisonneuve, V.; Lhoste, J. New Amorphous Iron-Based Oxyfluorides as Cathode Materials for High-Capacity Lithium-Ion Batteries. Journal of Physical Chemistry C 2019, 123 (35), 21386–21394. https://doi.org/10.1021/acs.jpcc.9b06055.
(2) Lemoine, K.; Zhang, L.; Dambournet, D.; Grenèche, J.-M.; Hémon-Ribaud, A.; Leblanc, M.; Borkiewicz, O. J.; Tarascon, J.-M.; Maisonneuve, V.; Lhoste, J. Synthesis by Thermal Decomposition of Two Iron Hydroxyfluorides: Structural Effects of Li Insertion. Chemistry of Materials 2019, 31 (11), 4246–4257. https://doi.org/10.1021/acs.chemmater.9b01252.
(3) Maisonneuve, V.; Lemoine, K.; Moury, R.; Lhoste, J.; Hémon-Ribaud, A.; Leblanc, M.; Grenèche, J.-M.; Tarascon, J.-M.; Tarascon, J.-M. Stabilization of a Mixed Iron Vanadium Based Hexagonal Tungsten Bronze Hydroxyfluoride HTB-(Fe0.55V0.45)F2.67(OH)0.33as a Positive Electrode for Lithium-Ion Batteries. Dalton Transactions 2020, 49 (24), 8186–8193. https://doi.org/10.1039/d0dt01310b.
(4) Chable, J.; Dieudonné, B.; Body, M.; Legein, C.; Crosnier-Lopez, M.-P.; Galven, C.; Mauvy, F.; Durand, E.; Fourcade, S.; Sheptyakov, D.; Leblanc, M.; Maisonneuve, V.; Demourgues, A. Fluoride Solid Electrolytes: Investigation of the Tysonite-Type Solid Solutions La1-XBaxF3-x (x < 0.15). Dalton Transactions 2015, 44 (45), 19625–19635. https://doi.org/10.1039/c5dt02321a.
(5) Dieudonné, B.; Chable, J.; Mauvy, F.; Fourcade, S.; Durand, E.; Lebraud, E.; Leblanc, M.; Legein, C.; Body, M.; Maisonneuve, V.; Demourgues, A. Exploring the Sm1-XCaxF3-x Tysonite Solid Solution as a Solid-State Electrolyte: Relationships between Structural Features and F- Ionic Conductivity. Journal of Physical Chemistry C 2015, 119 (45), 25170–25179. https://doi.org/10.1021/acs.jpcc.5b05016.
(6) Chable, J.; Martin, A. G.; Bourdin, A.; Body, M.; Legein, C.; Jouanneaux, A.; Crosnier-Lopez, M.-P.; Galven, C.; Dieudonné, B.; Leblanc, M.; Demourgues, A.; Maisonneuve, V. Fluoride Solid Electrolytes: From Microcrystalline to Nanostructured Tysonite-Type La0.95Ba0.05F2.95. Journal of Alloys and Compounds 2017, 692, 980–988. https://doi.org/10.1016/j.jallcom.2016.09.135.
(7) Dieudonné, B.; Chable, J.; Body, M.; Legein, C.; Durand, E.; Mauvy, F.; Fourcade, S.; Leblanc, M.; Maisonneuve, V.; Demourgues, A. The Key Role of the Composition and Structural Features in Fluoride Ion Conductivity in Tysonite Ce1−xSrxF3−x Solid Solutions. Dalton Transactions 2017, 46 (11), 3761–3769. https://doi.org/10.1039/c6dt04714a.
(8) POLTEAU B.; GOHARIBAJESTANI Z.; GUIET A.; LHOSTE J.; PASCUAL S.; MAISONNEUVE V.; BRUNET S.; HEMON-RIBAUD A.; GALVEN C. MATÉRIAUX FLUORURES INORGANIQUES MÉSOPOREUX. WO2020104758 (A1).
(9) Goharibajestani, Z.; Wang, Y.; Camus-Génot, V.; Arrii, S.; Comparot, J. D.; Polteau, B.; Lhoste, J.; Galven, C.; Gunes, V.; Hémon-Ribaud, A.; Pascual, S.; Body, M.; Legein, C.; Maisonneuve, V.; Brunet, S.; Guiet, A. MgF2-Based Organized Porous Inorganic Nanofluorides as Heterogeneous Catalysts for Fluorination of 2-Chloropyridine. ACS Applied Nano Materials 2021, 4 (10), 10601–10612. https://doi.org/10.1021/acsanm.1c01768.
(10) Lemoine, K.; Lhoste, J.; Hémon-Ribaud, A.; Heidary, N.; Maisonneuve, V.; Guiet, A.; Kornienko, N. Investigation of Mixed-Metal (Oxy)Fluorides as a New Class of Water Oxidation Electrocatalysts. Chemical Science 2019, 10 (40), 9209–9218. https://doi.org/10.1039/c9sc04027g.
(11) Maalej, O.; Boulard, B.; Dieudonné, B.; Ferrari, M.; Dammak, M.; Dammak, M. Downconversion in Pr3+–Yb3+ Co-Doped ZBLA Fluoride Glasses. Journal of Luminescence 2015, 161, 198–201. https://doi.org/10.1016/j.jlumin.2015.01.018.
(12) Maalej, O.; El Jouad, M.; Gaumer, N.; Chaussedent, S.; Boulard, B.; Ben Ameur, M. D.; Ben Tijani, M. D. Site Selection Spectroscopy in Eu3+-Doped Lanthanum Fluorozirconate Glass and Glass-Ceramic. Journal of Non-Crystalline Solids 2015, 420, 48–54. https://doi.org/10.1016/j.jnoncrysol.2015.04.025.
(13) Merigeon, J.; Maalej, O.; Boulard, B.; Stanculescu, A.; Leontie, L.; Mardare, D.; Girtan, M. Studies on Pr3+–Yb3+ Codoped ZBLA as Rare Earth down Convertor Glasses for Solar Cells Encapsulation. Optical Materials 2015, 48, 243–246. https://doi.org/10.1016/j.optmat.2015.08.008.
(14) Maalej, O.; Merigeon, J.; Boulard, B.; Girtan, M. Visible to Near-Infrared down-Shifting in Tm 3+ Doped Fluoride Glasses for Solar Cells Efficiency Enhancement. Optical Materials 2016, 60, 235–239. https://doi.org/10.1016/j.optmat.2016.07.047.
(15) Maalej, O.; Taktak, O.; Boulard, B.; Kammoun, S. Study with Analytical Equations of Absorption Spectra Containing Interference Dips in Fluoride Glasses Doped with Cr 3+. J. Phys. Chem. B 2016, 120 (30), 7538–7545. https://doi.org/10.1021/acs.jpcb.6b03230.
(16) Maalej, O.; Lukowiak, A.; Bouajaj, A.; Chiasera, A.; Righini, G. C.; Ferrari, M.; Boulard, B. Blue to NIR Down-Conversion in Tm3+/Yb3+-Codoped Fluorozirconate Glasses Compared to Pr3+/Yb3+ Ion-Pair. Journal of Luminescence 2018, 193, 22–28. https://doi.org/10.1016/j.jlumin.2017.09.021.
(17) Ravella, U. K.; Liu, J.; Corbel, G.; Skinner, S. J.; Lacorre, P. Cationic Intermixing and Reactivity at the La 2 Mo 2 O 9 /La 0.8 Sr 0.2 MnO 3− δ Solid Oxide Fuel Cell Electrolyte-Cathode Interface. ChemSusChem 2016, 9 (16), 2182–2192. https://doi.org/10.1002/cssc.201600516.
(18) Ravella, U. K.; Liu, J.; Corbel, G.; Skinner, S. J.; Lacorre, P. Cationic Interdiffusion at the SOFC Electrolyte/Cathode Interface in La 2 Mo 2 O 9 /La 0.8 Sr 0.2 MnO 3-δ. ChemistrySelect 2017, 2 (20), 5616–5623. https://doi.org/10.1002/slct.201701180.
(19) Sellemi, H.; Coste, S.; Barre, M.; Retoux, R.; Ben Ali, A.; Lacorre, P. Synthesis by the Polyol Process and Ionic Conductivity of Nanostructured La2Mo2O9 Powders. Journal of Alloys and Compounds 2015, 653, 422–433. https://doi.org/10.1016/j.jallcom.2015.07.250.
(20) Buvat, G.; Sellemi, H.; Ravella, U. K.; Barré, M.; Coste, S.; Corbel, G.; Lacorre, P. Reduction Kinetics of La 2 Mo 2 O 9 and Phase Evolution during Reduction and Reoxidation. Inorg. Chem. 2016, 55 (5), 2522–2533. https://doi.org/10.1021/acs.inorgchem.5b02876.
(21) Vega-Castillo, J.; Buvat, G.; Corbel, G.; Kassiba, A.; Lacorre, P.; Caneiro, A. On the Local Order of Amorphous La 2 Mo 2 O 6.7. Dalton Trans. 2017, 46 (22), 7273–7283. https://doi.org/10.1039/C7DT00637C.
(22) Buvat, G.; Quarez, E.; Joubert, O. Innovative Solid Oxide Fuel Cells Based on BaIn0.3Ti0.7O2.85 Electrolyte and La2Mo2O9 Amorphous Reduced Phase as Anode Material. Journal of Power Sources 2016, 302, 107–113. https://doi.org/10.1016/j.jpowsour.2015.10.030.
(23) Sabarthes, E.; Delorme, F.; Tezyk, V.; Autret, C.; Corbel, G.; Lacorre, P.; Giovannelli, F. Reducing the Thermal Conductivity of La 2 Mo 2 O 9 with a Trivalent Praseodymium Substitution for Its Potential Use as a Thermal Barrier Coating. Dalton Trans. 2019, 48 (27), 10051–10061. https://doi.org/10.1039/C9DT01192G.
(24) Emery, J.; Šalkus, T.; Abramova, A.; Barré, M.; Orliukas, A. F. NMR Investigations in Li 1.3 Al 0.3 Ti 1.7 (PO 4 ) 3 Ceramics. Part I: Structural Aspect. J. Phys. Chem. C 2016, 120 (46), 26173–26186. https://doi.org/10.1021/acs.jpcc.6b06764.
(25) Emery, J.; Šalkus, T.; Barré, M. NMR Investigations in Li 1.3 Al 0.3 Ti 1.7 (PO 4 ) 3 Ceramics Part II: Lithium Dynamics, Experiments, and Model. J. Phys. Chem. C 2016, 120 (46), 26235–26243. https://doi.org/10.1021/acs.jpcc.6b10392.
(26) Emery, J.; Šalkus, T.; Barré, M. NMR Investigations in Li 1.3 Al 0.3 Ti 1.7 (PO 4 ) 3 Ceramics Part III: Local Dynamical Aspect Seen from Aluminum and Phosphorus Sites. J. Phys. Chem. C 2017, 121 (1), 246–254. https://doi.org/10.1021/acs.jpcc.6b11712.
(27) Grenier, A.; Porras-Gutierrez, A. G.; Body, M.; Legein, C.; Chrétien, F.; Raymundo-Piñero, E.; Dollé, M.; Groult, H.; Dambournet, D. Solid Fluoride Electrolytes and Their Composite with Carbon: Issues and Challenges for Rechargeable Solid State Fluoride-Ion Batteries. Journal of Physical Chemistry C 2017, 121 (45), 24962–24970. https://doi.org/10.1021/acs.jpcc.7b07988.
(28) Li, W.; Corradini, D.; Body, M.; Legein, C.; Salanne, M.; Ma, J.; Chapman, K. W.; Chupas, P. J.; Rollet, A.-L.; Julien, C.; Zhagib, K.; Duttine, M.; Demourgues, A.; Groult, H.; Dambournet, D. High Substitution Rate in TiO 2 Anatase Nanoparticles with Cationic Vacancies for Fast Lithium Storage. Chemistry of Materials 2015, 27 (14), 5014–5019. https://doi.org/10.1021/acs.chemmater.5b01407.
(29) Li, W.; Body, M.; Legein, C.; Borkiewicz, O. J.; Dambournet, D. Atomic Insights into Nanoparticle Formation of Hydroxyfluorinated Anatase Featuring Titanium Vacancies. Inorganic Chemistry 2016, 55 (14), 7182–7187. https://doi.org/10.1021/acs.inorgchem.6b01259.
(30) Li, W.; Body, M.; Legein, C.; Dambournet, D. Sol-Gel Chemistry of Titanium Alkoxide toward HF: Impacts of Reaction Parameters. Crystal Growth and Design 2016, 16 (9), 5441–5447. https://doi.org/10.1021/acs.cgd.6b00910.
(31) Koketsu, T.; Ma, J.; Morgan, B. J.; Body, M.; Legein, C.; Dachraoui, W.; Giannini, M.; Demortière, A.; Salanne, M.; Dardoize, F.; Groult, H.; Borkiewicz, O. J.; Chapman, K. W.; Strasser, P.; Dambournet, D. Reversible Magnesium and Aluminium Ions Insertion in Cation-Deficient Anatase TiO2. Nature Materials 2017, 16 (11), 1142–1148. https://doi.org/10.1038/nmat4976.
(32) Li, W.; Body, M.; Legein, C.; Borkiewicz, O. J.; Dambournet, D. Solvothermal Temperature Drives Morphological and Compositional Changes through Dehydroxyfluorination in Anatase Nanoparticles. European Journal of Inorganic Chemistry 2017, 2017 (1), 192–197. https://doi.org/10.1002/ejic.201601160.
(33) Ma, J.; Koketsu, T.; Morgan, B. J.; Legein, C.; Body, M.; Strasser, P.; Dambournet, D. Controlled Hydroxy-Fluorination Reaction of Anatase to Promote Mg2+ Mobility in Rechargeable Magnesium Batteries. Chemical Communications 2018, 54 (72), 10080–10083. https://doi.org/10.1039/c8cc04136a.
(34) Ma, J.; Li, W.; Morgan, B. J.; Światowska, J.; Baddour-Hadjean, R.; Body, M.; Legein, C.; Borkiewicz, O. J.; Leclerc, S.; Groult, H.; Lantelme, F.; Laberty-Robert, C.; Dambournet, D. Lithium Intercalation in Anatase Titanium Vacancies and the Role of Local Anionic Environment. Chem. Mater. 2018, 30 (9), 3078–3089. https://doi.org/10.1021/acs.chemmater.8b00925.
(35) Koketsu, T.; Ma, J.; Morgan, B. J.; Body, M.; Legein, C.; Goddard, P.; Borkiewicz, O. J.; Strasser, P.; Dambournet, D. Exploiting Cationic Vacancies for Increased Energy Densities in Dual-Ion Batteries. Energy Storage Materials 2020, 25, 154–163. https://doi.org/10.1016/j.ensm.2019.10.019.
(36) Toma, O.; Leroux, M.; Mercier, N.; Allain, M.; Kassiba, A. H.; Swamy, S. K. K.; Dittmer, J. Bipyridinium‐bis(Carboxylate) Radical Based Materials: X‐ray, EPR and Paramagnetic Solid‐State NMR Investigations. Eur. J. Inorg. Chem. 2016, 2016 (7), 1036–1043. https://doi.org/10.1002/ejic.201501206.
(37) Toma, O.; Mercier, N.; Allain, M.; Kassiba, A. A.; Bellat, J.-P.; Weber, G.; Bezverkhyy, I. Photo- and Thermochromic and Adsorption Properties of Porous Coordination Polymers Based on Bipyridinium Carboxylate Ligands. Inorg. Chem. 2015, 54 (18), 8923–8930. https://doi.org/10.1021/acs.inorgchem.5b00975.
(38) Leblanc, A.; Mercier, N.; Allain, M.; Dittmer, J.; Fernandez, V.; Pauporté, T. Lead- and Iodide-Deficient (CH 3 NH 3 )PbI 3 ( d -MAPI): The Bridge between 2D and 3D Hybrid Perovskites. Angew. Chem. Int. Ed. 2017, 56 (50), 16067–16072. https://doi.org/10.1002/anie.201710021.
(39) Leblanc, A.; Mercier, N.; Allain, M.; Dittmer, J.; Pauporté, T.; Fernandez, V.; Boucher, F.; Kepenekian, M.; Katan, C. Enhanced Stability and Band Gap Tuning of α-[HC(NH 2 ) 2 ]PbI 3 Hybrid Perovskite by Large Cation Integration. ACS Appl. Mater. Interfaces 2019, 11 (23), 20743–20751. https://doi.org/10.1021/acsami.9b00210.