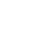(Bio)Hydrogels

Smart Hydrogels based on self assembled amphiphilic copolymers
By tempering the hydrophobic blocks of block and grafted amphiphilic copolymers (that is by incorporating hydrophilic subunits inside them), we have been able to obtain self-assemblies and hydrogels at thermodynamic equilibrium whereas non tempered amphiphilic block copolymers display mostly the formation of frozen self-assemblies. When the hydrophilic subunits are pH sensitive, the dynamics of the resulting hydrogels can be tuned in a reversible way with pH over 11 orders of magnitude. Dynamic self-assemblies can be blended easily to achieve mixed micelles and hydrogels of controlled properties through the stoechiometry of the blends. We can also achieve interpenetrated self-assembled networks responsive to various stimuli when mixing appropriate block copolymers. This approach has been exemplified with a great variety of amphiphilic copolymers.
Researchers
- Lazhar Benyahia
- Christophe Chasseneiux
- Olivier Colombani
- Erwan Nicol
- Taco Nicolaï
Related papers
- Tsitsilianis, C.; Serras, G.; Ko, C.-H.; Jung, F.; Papadakis, C. M.; Rikkou-Kalourkoti, M.; Patricicios, C. S.; Schweins, R.; Chassenieux, C. Thermoresponsive Hydrogels Based on Telechelic Polyelectrolytes: From Dynamic to "Frozen" Networks. Macromolecules 2018, 51, (6), 2169-2179. doi: 10.1021/acs.macromol.8b00193.
- Nicol, E.; Nicolai, T.; Zhao, J.; Narita, T. Photo-Cross-Linked Self-Assembled Poly(ethylene oxide)-Based Hydrogels Containing Hybrid Junctions with Dynamic and Permanent Cross-Links. Acs Macro Letters 2018, 7, 683-687. doi: DOI: 10.1021/acsmacrolett.8b00317.
- Lauber, L.; Santarelli, J.; Boyron, O.; Chassenieux, C.; Colombani, O.; Nicolai, T. pH- and Thermoresponsive Self-Assembly of Cationic Triblock Copolymers with Controlled Dynamics. Macromolecules 2017, 50, (1), 416-423. doi: 10.1021/acs.macromol.6b02201.
- Lauber, L.; Depoorter, J.; Nicolai, T.; Chassenieux, C.; Colombani, O. Viscoelastic Properties of Hydrogels Based on Self-Assembled Multisticker Polymers Grafted with pH-Responsive Grafts. Macromolecules 2017, 50, (20), 8178-8184. doi: 10.1021/acs.macromol.7b01585.
- Wright, D. B.; Patterson, J. P.; Gianneschi, N. C.; Chassenieux, C.; Colombani, O.; O'Reilly, R. K. Blending block copolymer micelles in solution; obstacles of blending. Polymer Chemistry 2016, 7, (8), 1577-1583. doi: 10.1039/c5py02006a.
- Lauber, L.; Colombani, O.; Nicolai, T.; Chassenieux, C. pH-Controlled Rheological Properties of Mixed Amphiphilic Triblock Copolymers. Macromolecules 2016, 49, (19), 7469-7477. doi: 10.1021/acs.macromol.6b01600.
- Chassenieux, C.; Tsitsilianis, C. Recent trends in pH/thermo-responsive self-assembling hydrogels: from polyions to peptide-based polymeric gelators. Soft Matter 2016, 12, (5), 1344-1359. doi: 10.1039/c5sm02710a.
- Wright, D. B.; Patterson, J. P.; Pitto-Barry, A.; Lu, A.; Kirby, N.; Gianneschi, N. C.; Chassenieux, C.; Colombani, O.; O'Reilly, R. K. The Copolymer Blending Method: A New Approach for Targeted Assembly of Micellar Nanoparticles. Macromolecules 2015, 48, (18), 6516-6522. doi: 10.1021/acs.macromol.5b01426.
- Wright, D. B.; Patterson, J. P.; Pitto-Barry, A.; Cotanda, P.; Chassenieux, C.; Colombani, O.; O'Reilly, R. K. Tuning the aggregation behavior of pH-responsive micelles by copolymerization. Polymer Chemistry 2015, 6, (14), 2761-2768. doi: 10.1039/c4py01782j.
- Lauber, L.; Chassenieux, C.; Nicolai, T.; Colombani, O. Highlighting the Role of the Random Associating Block in the Self-Assembly of Amphiphilic Block-Random Copolymers. Macromolecules 2015, 48, (20), 7613-7619. doi: 10.1021/acs.macromol.5b01626.
- Klymenko, A.; Nicolai, T.; Benyahia, L.; Chassenieux, C.; Colombani, O.; Nicol, E. Multiresponsive Hydrogels Formed by Interpenetrated Self-Assembled Polymer Networks. Macromolecules 2014, 47, (23), 8386-8393. doi: 10.1021/ma501990r.
Projects
- ANR GELLIGHT
- ANR FOGEL
- ANR MACAOs
- ANR DYNAMIC PISA
Fine-tuning of thermo-induced assembly and rheological behavior of hydrogels based on well-defined bis-hydrophilic block copolymer bearing a terpyridine entity by successive RAFT aqueous dispersion polymerization via metal ions equivalent and mixture.
Researchers
- Sagrario Pascual
- Sandie Piogé
- Lazhar Benyahia
- Laurent Fontaine
Related papers
- M. Le Bohec, M. Banère, S. Piogé, S. Pascual, L. Benyahia, L. Fontaine, Polym. Chem., 2016, 7, 6834
- A. Gutierres, S. Pascual, L. Fontaine, S. Piogé, L. Benyahia, Polym. Chem., 2018, 9, 2494
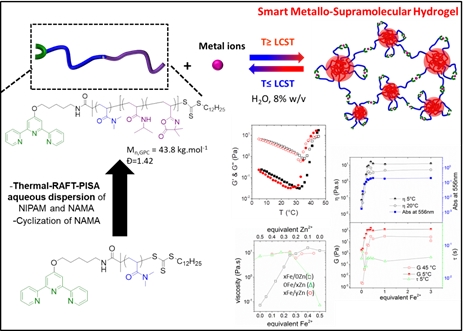
Innovative heterobifunctional azlactone-based linkers
Azlactone-based heterobifunctional linkers that proceed in orthogonal click-like reactions for chemical ligations in biologically relevant medium without releasing any by-product.
Researchers
- Sagrario Pascual
- Véronique Montembault
- Laurent Fontaine
Related papers
- Fontaine, H. T. Ho, S. Pascual, V. Montembault, PCT WO2014060357; US 20150285811A1; Japanese Patent n° 6262746 (2017)
- T. Ho, A. Bénard, G. Forcher, M. Le Bohec, V. Montembault, S. Pascual, L. Fontaine, Org. Biomol. Chem. 2018, 16, 7124-7128.
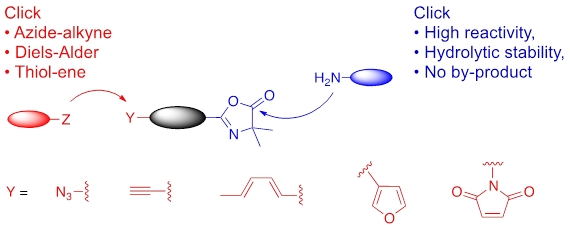
Innovative hetero-functional monomer: Vinylazlatone
Azlactone-derived polymers synthesized by controlled RAFT polymerization are able to react with amines according to the “click” chemistry concept: with full atom economy, quantitatively and with total chemoselectivity. Such feature leads to thermosensitive lysozyme bioconjugates, thermosensitive metallo-supramolecular flower-like micelles and antibody-functionalized magnetic nanoparticles.
Researchers
- Sagrario Pascual
- Sandie Piogé
- Véronique Montembault
- Lazhar Benyahia
- Laurent Fontaine
Related papers
- T.H. Ho, M.E. Levere, S. Pascual, V. Montembault, N. Casse, A. Caruso, L. Fontaine, Polym. Chem. 2013, 4, 675-685
- Delplace, S. Harrisson, H. T. Ho, J. A. Tardy, Y. Guillaneuf, S. Pascual, L. Fontaine, J. Nicolas, Macromolecules 2015, 48, 2087-2097
- Pray-In, C. Boonthip, B. Rutnakornpituk, U. Wichai, V. Montembault, S. Pascual, L. Fontaine, M. Rutnakornpituk, Materials Science & Engineering, C: Materials for Biological Applications 2016, 67, 285-293
- M. Le Bohec, M. Banère, S. Piogé, S. Pascual, L. Benyahia, L. Fontaine, Polym. Chem. 2016, 7, 6834