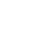Quaternary aminoacids

Quaternary aminoacids
Asymmetric Synthesis of QAA and incorporation into peptides
1,3-DC reactions between chiral aspartic nitrones and vinyl ethers provide isoxazolidinyl adducts able to be transformed into valuable bis-aspartic acid derivatives : ester (B) or aldehyde (C) :
The anhydride D efficiently produced from ester B acts as a synthetic platform for the generation of a variety of enantiopure QAA (E) featuring two extendable aspartic side chains,
From the aldehyde C, a robust pathway to the cyclic QAA derivative F was disclosed, which was found to act as a powerful beta-turn inducing agent when incorporated into a peptide.
Researchers
- Mathieu Laurent
- Arnaud Martel
- Gilles Dujardin
Related papers
- Zhang, P. Cividino, J.-F. Poisson, P. Shpak-Kraievskyi, M. Y. Laurent, A. Martel, G. Dujardin, S. Py Org. Lett., 2014, 16, 1936.
- Zhang, S. Chewchanwuttiwong, R. Hadade, M. Y. Laurent, A. Martel, J. Lhoste, S. Py, G. Dujardin submitted to J. Org. Chem.
Synthesis of symmetrical QAA
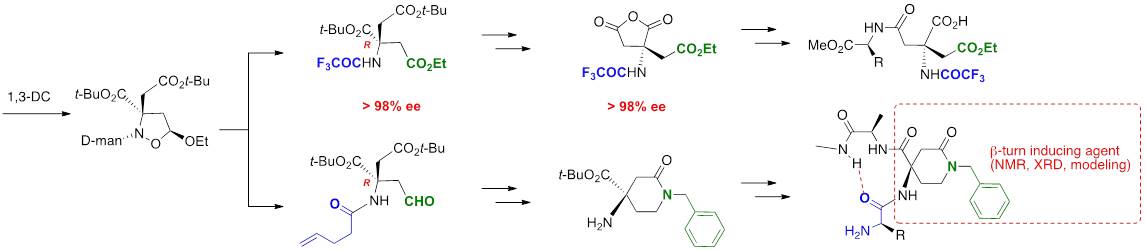
A straightforward synthesis of α,α-disubstituted α-amino acids was stiudied. The key step of this process relies on the efficient double addition of Grignard reagents to acyl cyanohydrins to provide N-acyl amino alcohols selectively in good yields. The chemoselectivity of the reaction was modulated by the nature of the acyl moiety. Different amino acids were prepared, including the particularly simple divinylglycine, which is not easily accessible by using conventional methods.
Researchers
- Fabien Boeda
- Morwenna Pearson-Long
- Philippe Bertus
Related papers
- A short Access to α,α-Disubstituted α-Amino Acids from Acyl Cyanohydrins
- Boukattaya, J. Caillé, H. Ammar, F. Rouzier, F. Boeda, M. S. M. Pearson-Long, P. Bertus Synthesis 2016, 48(6), 906-916.
Synthesis of Constrained Lysine Derivatives
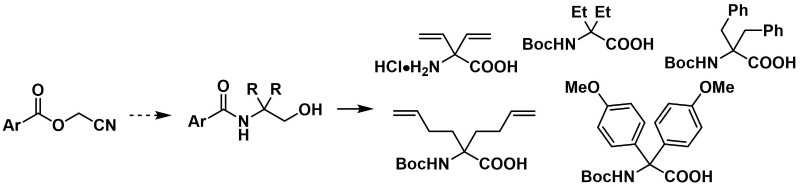
The Ti-mediated cyclopropanation reaction of nitriles using unsaturated Grignard reagents was found to be strongly dependent on the nature of the substrate and the solvent in terms of reactivity, yield and diastereoselectivity. The most useful result was obtained from benzyloxyacetonitrile and 4-pentenylmagnesium bromide, affording both the cis and the trans isomers in pure form. Ever if it is not diastereoselective, the simplicity of the method, using easily available substrates and reagents, and the easy separation of stereoisomers makes this method attractive for the preparation of constrained aminoacids. The synthetic utility of the obtained cyclopropylamines was thus illustrated by the synthesis of orthogonally protected 2,3-methanoaminoacids bearing an amino side chain with variable length, suitable for peptidic synthesis.
Researchers
- Fabien Boeda
- Morwenna Pearson-Long
- Philippe Bertus
Related papers
- Ti-Mediated Cyclopropanation of Nitriles with Unsaturated Grignard Reagents: Application to the Synthesis of Constrained Lysine Derivatives
- Forcher, N. Clousier, A. Beauseigneur, P. Setzer, F. Boeda, M. S. M. Pearson-Long, P. Karoyan, J. Szymoniak, P. Bertus Synthesis 2015, 47(7), 992-1006.
- F. Taber, Org. Chem. Highlights 2015, December 14.