Kidamycin

Kidamycin
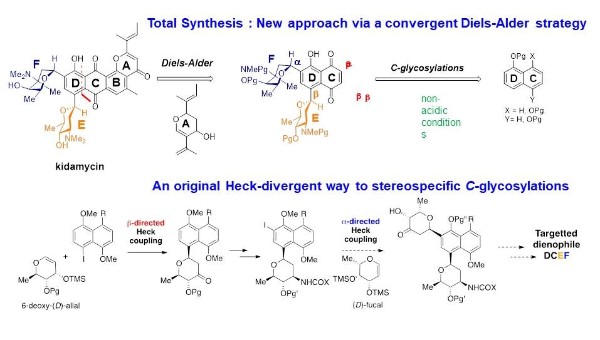
With partners of the ANR KidamySYN collaborative project, a new approach to kidamycine ( natural product, antitumoral and antibiotic activity, specific mode on interaction with DNA, no total synthesis reported to date) was investigated via a new strategy able to ensure the critical formation of the two C-glycosic links between the aromatic tetracyclic core ABCD and the amino sugars within the required anomeric configuration (alpha for E, beta for F).
Researchers
- Gilles Dujardin
- Arnaud Martel
- Christine Saluzzo
- Stéphane Guillarme
Related papers
- The convergent elaboration of the tetracycle ABCD by construction of the B cycle via a Diels-Alder was already validated in nor-sugar series : T. Mabit, A. Siard, M. Pantin, D. Zon, L. Foulgoc, D. Sissouma, A. Guingant, M. Mathe-́Allainmat, J. Lebreton, F. Carreaux, G. Dujardin, S. Collet. J. Org. Chem. 2017, 82, 5710-5719.
- The stereospecific implementation of the two amino-sugar unit to a CD platform is currently investigated using an innovative stereodivergent Heck strategy : T. Mabit, A. Siard, F. Legros, S. Guilllarme, A. Martel, J. Lebreton, F.Carreaux, G. Dujardin, S. Collet Chemistry – A European Journal, 2018 First published: 23 July 2018 https://doi.org/10.1002/chem.201803674